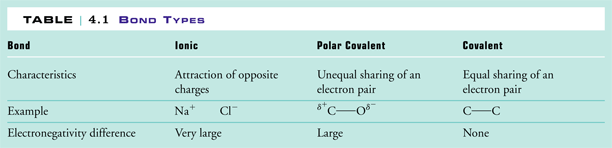
Distinguish between nonpolar covalent polar covalent and ionic bonds in terms of electron sharing. The atoms are now ionized or - and the bond is called ionic.
Unit 1 Elaboration Molecular Polarity
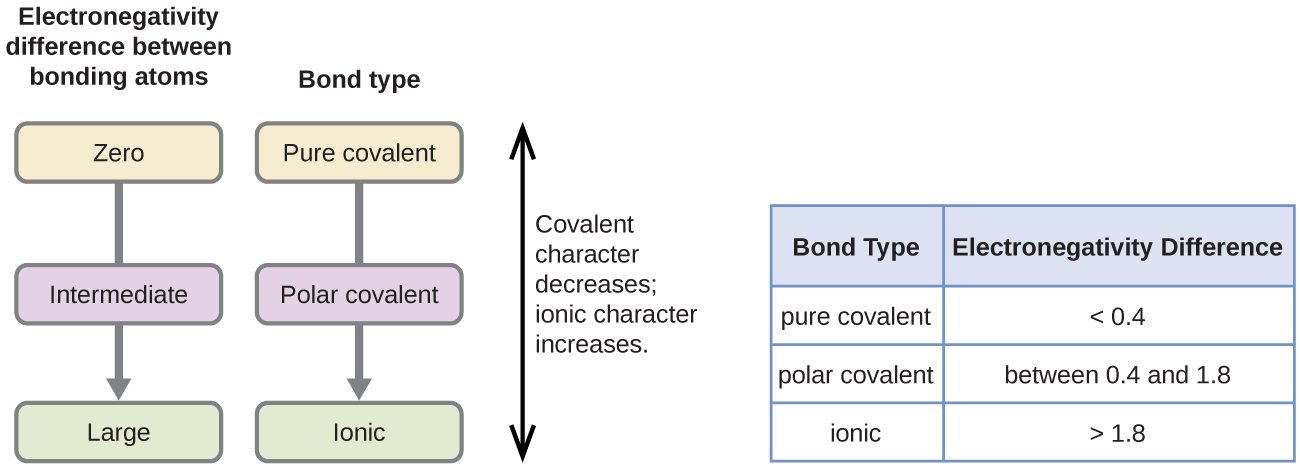
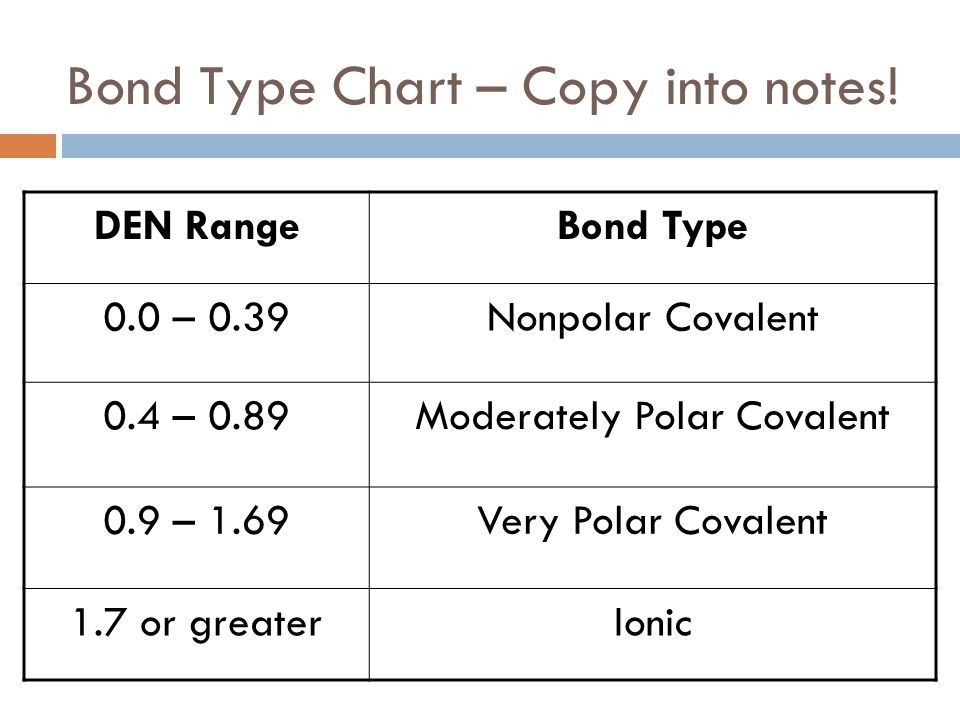
Somewhere between an electronegativity difference of 05 and 00 its a non-polar covalent.
. Both water and carbon dioxide have polar covalent bonds but carbon dioxide is linear so the partial charges on the molecule cancel each other out. Nonpolar Covalent Bonds Electrons are shared evenly when the two. Up to 24 cash back as a single unit.
Ionic or covalently bonded and to tape a paper label with the name and formula for each under the correct category sign on the board. EgCl 2 O 2 N 2 etc. Up to 24 cash back Polar and Nonpolar Covalent Bonds Chemical bonds exist along a continuum.
If youre electronegativity differences are between 17 and 05 then its a. Bonding between a metal and a nonmetal is often ionic. Water sugar vinegar ammonia or nonpolar symmetrical both ends of the molecule are the same.
Ionic Polar Covalent Non-Polar Covalent. The electronegativity value for oxygen is 344 whereas the electronegativity value for hydrogen is 220. Chemistry 1011 Slot 5 3 Other Materials There are also other materials with complex.
Are the bonds in ammonia polar covalent or nonpolar covalent. Because of the close sharing of pairs of electrons one electron from each of two atoms covalent bonds are stronger than ionic bonds. Table 1 nonpolar covalent polar covalent ionic Electronegativity Difference E 000 065 094 119 143 167 191 219 254 303.
More than one type of atom bonded together Ionic bonding Metallic bonding. This is shown in Table 1. Type of bonding in which an atom loses or gains electrons.
However these polyatomic ions form ionic compounds by. The polarity and Non-polarity of molecules depend upon electronegativity. Water is a highly polar substance.
Determine the polarity of a chemical bond using the electronegativity chart. Polar bonds have high melting point surface tension boiling point and low vapour pressure. There are two types of covalent bonds.
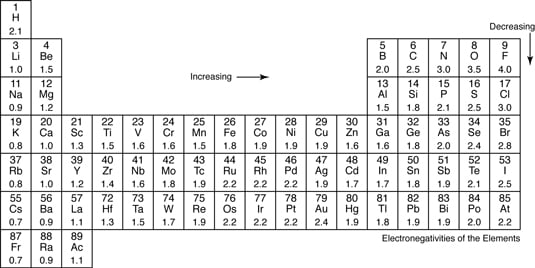
The greater the difference in electronegativity between two atoms the more polar their bond is. Difference in EN 2 CH bonds relatively nonpolar C-O C-X bonds more electronegative elements are polarelectronegative elements are polar Bonding electrons shift toward. Negative and the less electronegative atom to be.
H 2 O HI HCl NH 3. When will a bond be polar. Explain In a molecule of ammonia NH 3 three hydrogen atoms are attached to one nitrogen atom.
Covalent bonds formed between atoms of the same kind are usually non-polar and the shared electrons are equidistant from both atomic nuclei. The difference in the distribution of electrons accounts for the best shape of the molecule. Whether a molecule is polar or nonpolar depends both on bond type and molecular shape.
The atoms in polyatomic ions such as OH NO 3 NO 3 and NH 4 NH 4 are held together by polar covalent bonds. Remind the class that covalent substances are either polar not symmetrical the molecule has two different ends. F F D en 0 nonpolar covalent H F D en 19 polar covalent LiF D en 30 ionic Ionic Bonds Polar Covalent Bonds Nonpolar Covalent Bonds.
Ionic compounds are made up of ions positively charged cations and negatively charged anions and thus there is a strong electrostatic force of attraction between these ions and so they are hard solids. Not bonded to another type of atom Compound. Network covalent solids Ionic solids Metallic solids.
Notice that the two covalently bonded atoms typically share just. Bond Polarity and Inductive EffectBond Polarity and Inductive Effect Nonpolar Covalent Bonds. As an example consider the bond that occurs between an atom of potassium and an atom of fluorine.
Bonds can be found with a range of polarities from completely ionic to completely covalent. Examples of Molecules with Polar Covalent Bonds. A property of molecules in which one or more atoms possess either a partial positive or a partial negative charge.
The oxygen side of the molecule features. Non-polar covalent bond Formed by identical atoms. Atoms with similar EN Polar Covalent Bonds.
Type of covalent bond that involves two pairs of electrons. Covalent compounds are usually not bad conductors of electricity. Back to first slide.
Water H2O is a molecule having a polar covalent bond. HBr is a polar covalent bond. Using the table the difference in electronegativity is 40 08 32.
Some compounds contain both covalent and ionic bonds. Chemistry 1011 Slot 5 13 Attractions Between Ions The force of attraction between oppositely charged ions depends on. A polyatomic ion can form ionic bonds with other polyatomic ions or with monatomic ions.
The atoms seek to fill their outermost shell with eighteen electrons. Chemical bonds exists as polar covalent bonds and nonpolar covalent bonds. Then as the sharing becomes more and more unequal the electrons reside only on one atom.
Instead the electrons move back and forth between the elements. 7 rows Bonds that are partly ionic are called polar covalent bonds. Figure 2 shows several common types of covalent bonds.
Bonds between two nonmetals are generally covalent. Polar Non-polar Solubility Polar and Nonpolar Covalent Bonds. Unequal sharing of electrons causes the more electronegative atom of the bond to be.
The degree to which a given bond is ionic or covalent is determined by calculating the difference in electronegativity between the two atoms involved in the bond. Polar covalent bond Formed between atoms having a difference in their electronegativities. 5 Polar Covalent Bond This is a type of covalent bond.
Solubility tests are best for distinguishing between molecular polar and nonpolar substances Ionic substances have the strongest bonds ionic bonds and therefore have the highest melting points 124. Water is an example of a molecule that has polar covalent bonds and engages in hydrogen bonding. The atoms electronegativity difference is greater than 04.
The more electronegative an atom the more it seeks electronsIf one atom is more electronegative than others it can form an ionic bond or a polar covalent bond. The type of bond in which electrons are shared between two atoms. The Strongest force and forms H bonds or dipole- dipole bonds.
Difference in EN of atoms 2 Ionic Bonds. Because the teflon molecules are non-polar and dont readily become induced dipoles few things stick to teflon. In Biological systems polar covalent bonds are important because they allow the formation of another kind of weak bond called a hydrogen bond.
The theory of electronegativity lies in entire inorganic chemistry. A covalent bond in which the shared electron pair is not shared equally but remains closer to one atom than the other is a.
7 2 Covalent Bonding Chemistry
Chemystery Electronegativity And Polarity
Solved This Question Is Already Answered The Signs In Blue Chegg Com
0 Comments